Sodium Fluoride Is Used as an Anticoagulant for Which Chemistry
This anti-coagulant is used for preparing blood specimens for the determination of glucose and urea in plasma by non-enzymatic methods. Oxalate and EDTA anticoagulants will give plasma samples.

Anticoagulants Used In Haematology
Sodium fluoride is used in trace amounts in the process of fluoridation of drinking water toothpaste and also in the pesticides.

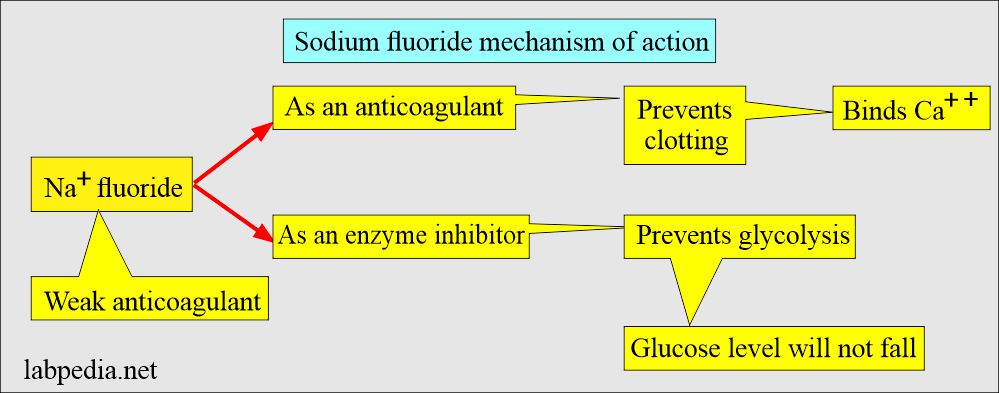
. This is a weak anticoagulant but uses an antiglycolytic agent to preserve glucose. Sodium Fluoride Mechanism of Action. Sodium Fluoride - It is NOT an anticoagulant but a preservative with antiglycolytic property - Prevents glycolysis of glucose for 72 hours - Available in GRAY-top tube - If the gray-top tube is labeled with Sodium fluoride only then it will yield a SERUM sample - If plasma sample is needed an anticoagulant must be.
Tube inversions ensure mixing of anticoagulant heparin with blood to prevent clotting. As an anticoagulant by binding the calcium. Should always be facing up when inserted into the skin.
Chemistry testing especially glucosesugar and lactate Glucose tolerance test GTT 7. Sodium fluoride is the anticoagulant choice for Blood cells are suspended in the plasma i m sorry is made up of water and dissolved materials consisting of hormones antibodies and enzymes that are being lugged to the tissues and also cellular waste commodities that are being carried to the lungs and also kidneys. However in fluoridated non-separated blood samples glucose is still metabolized at approximately 5 to 7 per hour at room temperature because upstream enzymes continue to convert it to glucose-6.
When plasma is required for blood chemistry analysis the anticoagulant of choice is. You are watching. - NaHep is the injectable form used for anticoagulant therapy c.
Sodium or lithium heparin. It acts in two ways. The anticoagulant used to preserve blood glucose levels for blood chemistry analysis is.
Product name sodium fluoride NaF Molecular weight 4199. The anticoagulant the sodium fluoride in a higher concentration can also act as a preservative. The two substances that would be used could be one either sodium fluoride or whats know as sodium editate PHONETIC or it is abbreviated sodium edta one acts as an anticoagulant and one acts more as a preservative.
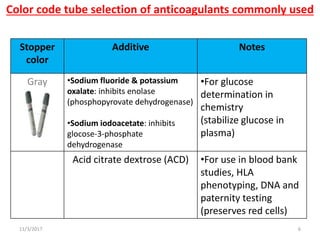
Sodium fluoride is a chemical that consists of a combination of positively charged sodium ions and negatively charged fluoride ions. Chemistry Anticoagulant Contamination and Anticoagulant Additives. Gray Gray Potassium oxalate sodium fluoride Sodium fluorideNa 2 EDTA Sodium fluoride serum tube 8 8 8 For glucose determinations.
Increased Potassium K with Hemolysis should only go up max 30 anything over 13 is impossible Sodium Fluoride Potassium Oxalate. Properties White powder toxic. Sodium fluoride acts as an antiglycolytic agent to ensure that no further glucose breakdown occurs within the sample after it is taken.
It binds with the calcium ions present in the blood and forms Calcium fluoride. Gray stopper contains the anticoagulant sodium fluoride used in chemistry for glucose testing and alcohol testing. This inhibits the system involved in glycolysis and preserves the glucose.
Used to collect plasma contains the anticoagulant sodium heparin used in chemistry for STAT testing. Sodium Fluoride antiglycolytic and Potassium Oxalate anticoagulant Laboratory. Potassium oxalate removes calcium and acts as an anticoagulant.
Worksheet List of Common Blood Additives Anticoagulants Additive Color Code Use Chemical Reactions Sodium Fluoride and Sodium or Potassium Oxalate Gray Fluoride inhibits glycolysis and oxalate prevents clotting by calcium. Fluroide inhibits glycolic enzymes and thereby prevents loss of glucose during transportation or delay in specimen handling. Both sodium and fluoride are essential minerals.
Sodium hydroxide is a strong base and hydrogen fluoride is a weak acid so sodium fluoride would be weakly basic. Glass Sodium Fluoride Potassium Oxalate Gray-TopWHOLE BLOOD PLASMA. Executive standard GBT1264-1997 national standard.
Sodium fluoride Table 1 inhibits the glycolytic enzyme enolase and is used to limit the ex vivo consumption of glucose by cells in a collected blood specimen. Contains potassium oxalate as an anticoagulant and sodium fluoride as a preservative used to preserve glucose in whole blood and for some special chemistry tests as well as lactic acid testing. As fluoride is not a strong anticoagulant it is mixed with oxalate.
What additive does. I would have to look at the tubes in this kit to see which or if. Quality control sera used in most laboratories are supplied in what form.
For plasma determinations in chemistry. Slows coagulation and prevents new clots from forming. Process of blood clotting.
Sodium fluoride acts as an antiglycolytic agent to ensure that no further glucose breakdown occurs within the sample. Decreased Magnesium Calcium Sodium Citrate. Toothpaste is composed of the tin fluoride and.
It is the anticoagulant of choice for the estimation of blood sugar. It exists as a white powder that easily dissolves in water. Therefore the correct option is option D.
The National Institutes of Medicine Food and Nutrition Board recommends adults take in 3 mg of fluoride daily. The complete process of storing and preserving blood requires measures such as storage in proper bags separation of different components of blood and use of proper anticoagulants. Soluble in water the solution is corrosive to glass.
Almost insoluble in ethanol. The Calcium Oxalate precipitate that forms in the blood is harmful as it is a toxic agent it is not used as a preservative in blood banks. Sodium fluoride is the.
This can be used as a dry additive. Chemical name of toothpaste. AR Grade Sodium Fluoride Powder NaF Anticoagulant Used In Blood Transfusion.
Decreased Calcium Magnesium.

Anticoagulant Anticoagulant Is A Substance That Prevents Blood

Effect Of Hexokinase And Enolase Inhibition In The Glycolytic Pathway Download Scientific Diagram
Anticoagulants Used In Haematology
Anticoagulants Used For Routine Tests Principle Preparation Uses

Pdf Effectiveness Of Sodium Fluoride As A Preservative Of Glucose In Blood

Pin By Joy Benedict On Next Vid Phlebotomy Study Medical Student Study Clinical Chemistry

Medical Advice For Wine Pin Medical Medical Advice Red Wine
Effect Of Anticoagulant Type During The Blood Sample Collection Process On The Determination Of The Acylcarnitine Profile In Blood And Plasma
Pdf Effects Of Common Anticoagulants Heparin Citrate And Edta On Routine Plasma Biochemistry Of Cattle

Order Of Draw Poster Draw Order Poster Order Of Draw Medical Technology Medical Marketing

Anticoagulants Used In Haematology

Pdf Influence Of K2edta Sodium Citrate And Lithium Heparin Anticoagulants On Lipid Profile In Plasma Samples As Measured By An Automated Chemical Analyzer

Clinical Chemistry Review Sheet For Mlt Certification And Ascp Clinical Chemistry Chemistry Review Medical Laboratory Science





Comments
Post a Comment